DOH Medicaid Update September 2006 Vol. 21, No. 9
Office of Medicaid Management
DOH Medicaid Update
September 2006 Vol. 21, No. 9
State of New York
George E. Pataki, Governor
Department of Health
Antonia C. Novello, M.D., M.P.H., Dr. P.H.
Commissioner
Medicaid Update
is a monthly publication of the
New York State Department of Health,
Office of Medicaid Management
Brian J. Wing, Deputy Commissioner
Table of Contents
Helpful Prior Authorization Tips for Prescribers
Helpful Tips for Pharmacists When Validating Prior Authorization Numbers
New York State Preferred Drug List
New York State Preferred Drug Quick List
Integrated Voice Response System for Prior Authorization Programs
Official Prescription Program Update
Return of Unused Medications from Nursing Facilities
Reimbursement for Automated Urinalysis in a Practitioner's Office
Payment for Speech Generating Devices in Facilities
Prepayment Review Will End for Some Procedures
Krabbe Disease: Payment for Testing and Treatment
New Process for Orthodontic Prior Approval for Nassau and Suffolk Counties Effective September 1, 2006
Clarification of Medicaid Coverage for Physical Examinations and Travel Immunizations
Proper Billing Requirements for Clinics
Certain Long Term Therapy Services Incorporated into ICF/DD Medicaid Payment Rates Effective January 1, 2007
Coverage of Specialist Consultations via Telemedicine
Annual Recertification for Providers Submitting Electronic Claims
Patient Education: Prevent Disease Immunize Please
Long Term Home Health Care Program Reference Manual
Medicaid Requires Providers to Stay Informed of Policy and Claiming Rules
Attention Rate Based Institutional Providers: Disclosure of Ownership Document
Citizenship Documentation Requirements of the Deficit Reduction Act of 2005 Effective July 1, 2006
New York State Medicaid Online Addresses
Provider Services
Pharmacy Prior Authorization Changes
Effective October 18, 2006
Return to Table of Contents
Prior Authorization Process
Effective October 18, 2006, all pharmacy prior authorizations will be initiated by calling the centralized Clinical Call Center at:
(877) 309-9493.
Live operators are available for all prior authorization requests other than the Mandatory Generic Program. Other changes will include:
- Serostim and Zyvox prior authorization process will be moved from the current electronic voice interactive phone system (VIPS) to the staffed Clinical Call Center.
- Prior authorization for Revatio will now be handled through the staffed Clinical Call Center as well, rather than the special billing process now in place.
- Several new drug categories will be added to the Preferred Drug Program (PDP). Please refer to the Quick List below.
- Second generation prescription antihistamines and proton pump inhibitors will be transferred into the PDP. (This change includes the availability of additional proton pump inhibitors which may be prescribed without prior authorization, so please review this information carefully.)
The Clinical Drug Review Program
Return to Table of Contents
The Medicaid Clinical Drug Review Program (CDRP) is aimed at ensuring specific drugs are utilized in a medically appropriate manner.
Serostim and Zyvox, which currently require prior authorization through the voice interactive phone system (VIPS), will transition to the CDRP and will be prior authorized through the staffed Clinical Call Center, effective October 18, 2006.
Affected Drugs
Prescriptions written on or after October 18, 2006 for the following drugs will require prior authorization under the CDRP:
- Revatio
- Serostim
- Zyvox
Prior Authorization Process
Under the CDRP, only the prescriber, not the authorized agent, can initiate the prior authorization process by calling the staffed prior authorization call center at:
(877) 309-9493 and listening for the appropriate prompts.
A pharmacy technician or a pharmacist will ask for specific clinical information intended to demonstrate the patient's medical need for the CDRP drug. Once authorization is given and a prior authorization number is obtained, the number must be written on the face of the prescription.
Pharmacists are required to validate the prior authorization number prior to dispensing. To validate the prior authorization, call:
(877) 309-9493 and listen for the appropriate prompts.
Confirmation that there is authorization to dispense the drug will be provided. The prior authorization number must be entered into the prior authorization code field when billing.
Additional information, such as updated prior authorization forms, the clinical criteria that must be met for authorization and receiving authorization for an emergency supply is available at:
www.nyhealth.gov or http://newyork.fhsc.com

Questions
If you have questions, please call:
(877) 309-9493 and select Option "3" for technical assistance.
Expansion of the Preferred Drug Program
Return to Table of Contents
Effective October 18, 2006, several new drug categories will be added to the current list of drugs subject to the Preferred Drug Program (PDP).
Affected Categories

New categories being added to the PDP are Second Generation Prescription Antihistamines (SGA) and Proton Pump Inhibitors (PPI), which currently require prior authorization through the voice interactive phone system.
Prior Authorization Process
Prescribers are required to complete the prior authorization process for their patients to receive non-preferred drugs.
Prior authorization of prescriptions for non-preferred drugs written on or after October 18, 2006 in either of these categories must be obtained through the staffed Clinical Call Center.
Drugs identified as "preferred" by the New York State Medicaid Program do not require prior authorization.
To obtain prior authorization for a non-preferred drug, contact the prior authorization Clinical Call Center at:
(877) 309-9493 and listen for the appropriate prompts.
Preferred Drug List
Listed below in this Medicaid Update is the full Preferred Drug List (PDL) showing preferred and non-preferred drugs.
Also included below is the complete Quick List, which is an easy-to-use summary of preferred drugs in each of the PDP categories.
Please print these sheets and keep them handy.
If you prescribe the preferred drug, no prior authorization is necessary.
Note: Additional prescription proton pump inhibitors are now available without prior authorization.
Additional information is available at www.nyhealth.gov or http://newyork.fhsc.com
Helpful Prior Authorization Tips
for Prescribers
Return to Table of Contents
Effective October 18, 2006, technical assistance for all pharmacy prior authorizations will be provided through the Prior Authorization Clinical Call Center.
Below are some helpful hints for obtaining prior authorizations and for receiving technical assistance with all pharmacy prior authorizations.
Prescriber: Important Prior Authorization Phone Numbers
To get a PA (Prior Authorization) for the Mandatory Generic Program (brand name drugs):
- Call (877) 309-9493
- Select OPTION "1" for PRESCRIBER
- Then select OPTION "2" for BRAND NAME DRUGS
To get a PA (Prior Authorization) for the PDP (Preferred Drug Program) and the CDRP (Clinical Drug Review Program:
- Call (877) 309-9493
- Select OPTION "1" for PRESCRIBER
- Then select OPTION "1" for ALL OTHER DRUGS
For technical assistance with any of the above pharmacy prior authorization programs:
- Call (877) 309-9493
- Select OPTION "1" for PRESCRIBER
- Then select OPTION "3" for TECHNICAL ASSISTANCE
Helpful Prior Authorization Tips
for Pharmacists
When Validating Prior Authorization Numbers
Return to Table of Contents
Effective September 28, 2006, technical assistance for all pharmacy prior authorizations will be provided through the Prior Authorization Clinical Call Center.
Here are some helpful hints for validating prior authorization and for receiving technical assistance with all pharmacy prior authorizations.
Pharmacy: Important Prior Authorization Phone Numbers
To validate a PA (Prior Authorization) for the Mandatory Generic Program (PA numbers without a "W" at the end):
- Call (877) 309-9493
- Select OPTION "2" for PHARMACY
- Then select OPTION "2" for BRAND NAME DRUGS
To validate a PA (Prior Authorization) for the Preferred Drug Program and the Clinical Drug Review Program (PA numbers with a "W" at the end):
- Call (877) 309-9493
- Select OPTION "2" for PHARMACY
- Then select OPTION "1" for ALL OTHER DRUGS
For technical assistance with any of the above pharmacy prior authorization programs:
- Call (877) 309-9493
- Select OPTION "2" for PHARMACY
- Then select OPTION "3" for TECHNICAL ASSISTANCE
To discuss POS issues, billing problems such as payment questions or errors in pharmacy claims:
Call: (800) 343-9000.
New York State Medicaid Preferred Drug List
All non-preferred drugs in these classes will require prior authorization.
| ACE Inhibitors | ACE Inhibitors |
|---|---|
| PREFERRED AGENTS | NON-PREFERRED AGENTS - PA Required Effective 6/28/06 |
| Altace® benazepril captopril enalapril maleate lisinopril Mavik® moexipril |
Accupril® Aceon® Capoten® fosinopril sodium Lotensin® Monopril® Prinivil® quinaprill Univasc® Vasotec® Zestril® |
| ACE Inhibitors + Calcium Channel Blocker | ACE Inhibitors + Calcium Channel Blocker |
| PREFERRED AGENTS | NON-PREFERRED AGENTS - PA Required Effective 6/28/06 |
| Lotrel ® Tarka® |
Lexxel® |
| ACE Inhibitors + Diuretic | Ace Inhibitors + Diuretic |
| PREFERRED AGENTS | NON-PREFERRED AGENTS - PA Required Effective 6/28/06 |
| benazepril/HCTZ captopril/HCTZ enalapril maleate/HCTZ lisinopril/HCTZ Unieretic® |
Accuretic® Capozide® fosinopril HTC Lotensin HCT® Monopril HCT® Prinzide® quinapril/HCTZ Quinaretic® Vaseretic® Zestoretic® |
| Angiotensin Receptor Blockers | Angiotensin Receptor Blockers |
| PREFERRED AGENTS | NON-PREFERRED AGENTS - PA Required Effective 6/28/06 |
| Benicar®® Cozaar® Diovan® Micardis ® |
Atacand® Avapro® Teveten® |
| Angiotensin Receptor Blocker + Diuretic | Angiotensin Receptor Blocker + Diuretic |
| PREFERRED AGENTS | NON-PREFERRED AGENTS - PA Required Effective 6/28/06 |
| Benicar HTC® Diovan HTC® Hyzaar® Micardis HCT® | Atacand HCT® Avalide® Teveten HCT® |
| Anti-Emetics - Oral | Anti-Emetics - Oral |
| PREFERRED AGENTS | NON-PREFERRED AGENTS - PA Required Effective 10/18/06 |
| Kytril®(tablet, solution) Zofran®(tablet, solution, ODT) |
Anzemet® |
| Second Generation Antihistamines | Second Generation Antihistamines CC |
| PREFERRED AGENTS | NON-PREFERRED AGENTS - PA Required Effective 10/18/06 |
| OTC loratadine® OTC loratadine-D | Allegra® Allegra-D® Clarinex® Clarinex-D® fexofenadine Semprex-D® Zyrtec®CC Zyrtec-D® |
| Beta Blockers | Beta Blockers |
| PREFERRED AGENTS | NON-PREFERRED AGENTS - PA Required Effective 6/28/06 |
| acebutolol atenolol betaxolol bisoprolol funerate labetalol metoprolol tartrate nadolol pindolol propranolol timolol maleate |
Blocadren® Coreg®CC Corgard® Inderal LA® Inderal® InnoPran XL® Kerolone® Lopressor® Levatol® Sectral® Tenormin® Toprol XL®CC Trandate® Zebeta® |
| Beta Blocker + Diuretic | Beta Blocker + Diuretic |
| PREFERRED AGENTS | NON-PREFERRED AGENTS - PA Required Effective 10/18/06 |
| atenolol/chlorthalidone bisoprolol funerate/HCTZ metoprolol tartrate/HCTZ propranolol/HCTZ |
Corzide® Inderide® Inderide LA® Lopressor HCT® Tenoretic® Timolide® Ziac® |
| Bisphosphonates - Oral | Bisphosphonates - Oral |
| PREFERRED AGENTS | NON-PREFERRED AGENTS - PA Required Effective 6/28/06 |
| Fosamax®(tablet, solution) Fosamax®Plus D |
Actonel® Actonel®with Calcium Boniva® |
| Calcitonins - Nasal | Calcitonins - Nasal |
| PREFERRED AGENTS | NON-PREFERRED AGENTS - PA Required Effective 10/18/06 |
| Miacalcin® | Fortical® |
| Calcium Channel Blockers (DHP) | Calcium Channel Blockers (DHP) |
| PREFERRED AGENTS | NON-PREFERRED AGENTS - PA Required Effective 6/28/06 |
| Afeditab CR® Dynacirc ® Dynacirc CR® felodipine ER isradipine nicardipine HCL Nifediac CC® Nifedical XL® nifedipine nifedipine ER nifedipine SA Norvasc® Sular® |
Adalat CC® Cardene® Cardene SR® Plendil® Procardia® Procardia XL® |
| Hepatitis C Agents | Hepatitis C Agents |
| PREFERRED AGENTS | NON-PREFERRED AGENTS - PA Required Effective 10/18/06 |
| PEG-Intron® PEG-Intron Redipen® Pegasys® Pegasys Convenience Pack® | None |
| HMG-CoA Reductase Inhibitors (Statins) | HMG-CoA Reductase Inhibitors (Statins) |
| PREFERRED AGENTS | NON-PREFERRED AGENTS - PA Required Effective 10/18/06 |
| Advicor® Altoprev® Crestor® Lescol® Lescol XL Lipitor® Vytorin® Zocor® |
Caduet® lovastatin Mevacor® Pravachol® prevastatin PravigardPAC® |
| Leukotriene Modifiers | Leukotriene Modifiers |
| PREFERRED AGENTS | NON-PREFERRED AGENTS - PA Required Effective 10/18/06 |
| Accolate® Singular® | None |
| Narcotics - Long Acting | Narcotics - Long Acting |
| PREFERRED AGENTS | NON-PREFERRED AGENTS - PA Required Effective 10/18/06 |
| Duragesic® fentanyl patch Kadian® morphine sulfate SR Oramorph SR® |
Avinza® MS Contin® oxycodone HCL CR Oxycontin® |
| Proton Pump Inhibitors | Proton Pump Inhibitors |
| PREFERRED AGENTS | NON-PREFERRED AGENTS - PA Required Effective 10/18/06 |
| Nexium® Prevacid®(capsule) Prilosec®OTC |
Acifex® omeprazole Prevacid NapraPAC® Prevacid® (solutab, suspension) Prilosec® Protonix® Zegerid® (capsule, packet) |
| Sedative Hypnotics/Sleep Agents | Sedative Hypnotics/Sleep Agents |
| PREFERRED AGENTS | NON-PREFERRED AGENTS - PA Required Effective 10/18/06 |
| Ambien CR® chloral hydrate estazolam flurazepam temazepam triazolam |
Ambien® Dalmane® Doral® Halcion® Lunesta® Prosom® Restoril® Rozerem® Somnote® Sonta® |
| Serotonin Receptor Agonists (Triptans) | Serotonin Receptor Agonists (Triptans) |
| PREFERRED AGENTS | NON-PREFERRED AGENTS - PA Required Effective 10/18/06 |
| Imitrex®(tablet, nasal, injection) Maxalt®(tablet, MLT) | Amerge® Axert® Frova® Relpax® Zomig®(tablet, nasal, ZMT) |
| Steroids - Intranasal | Steroids - Intranasal |
| PREFERRED AGENTS | NON-PREFERRED AGENTS - PA Required Effective 10/18/06 |
| Nasacort AQ® Nasonex® |
Beconase AQ® Flonase® flunisolide® fluticasone® Nasarel® Rhinacort Aqua® |
| Thiazolidinediones | Thiazolidinediones |
| PREFERRED AGENTS | NON-PREFERRED AGENTS - PA Required Effective 10/18/06 |
| Actos® Actoplus met® Avandia® Avandamet® Avandaryl | None |
| Triglyceride Lowering Agents | Triglyceride Lowering Agents |
| PREFERRED AGENTS | NON-PREFERRED AGENTS - PA Required Effective 10/18/06 |
| gemfibrozil Lofirba® |
Antara® fenofibrate® Lopid® Omacor® Tricor Triglide |
CC-Clinical Criteria
Please see: https://newyork.fhsc.com/downloads/providers/NYRx_PDP_clinical_criteria.pdf
New York State Medicaid Preferred Drug "Quick List" - Phase I and II
Return to Table of Contents
These drugs are preferred and do not require prior authorization.
| ACE Inhibitors |
|---|
| PREFERRED AGENTS |
| Altace® benazepril captopril enalapril maleate lisinopril Mavik® moexipril |
| ACE Inhibitors + Diuretic |
| PREFERRED AGENTS |
| benazepril/HCTZ captopril/HCTZ enalapril maleate/HCTZ lisinopril/HCTZ Unitretic® |
| Angiotensin Receptor Blockers + Diuretic |
| Benicar HCT® Diovan HCT® Hyzaar® Micardis HCT® |
| Antihistamines - Second Generation |
| PREFERRED AGENTS |
| OTC loratadine OTC loratadine-D |
| Beta Blockers + Diuretic |
| PREFERRED AGENTS |
| atenolol/chlorthalidone bisoprolol fumerate HCTZ metoprolol tartrate/HCTZ propranolol/HCTZ |
| Calcitonins - Nasal |
| PREFERRED AGENTS |
| Miacalcin® |
| Hepatitis C Agents |
| PREFERRED AGENTS |
| PEG-Intron® PEG-Intron Redipen Pegasys® Pegasys Convenience Pack® |
| Leukotriene Modifiers |
| PREFERRED AGENTS |
| Accolate® Singular |
| Proton Pump Inhibitors |
| PREFERRED AGENTS |
| Nexium® Prevacid®(capsule) Prilosec®OTC |
| Serotonin Receptor Agonists (Triptans) |
| PREFERRED AGENTS |
| Imitrex®(tablet, nasal, injection) Maxalt®(tablet, MLT) |
| Thiazolidinediones |
| Actos® Actoplus met® Avandia® Avandamet® Avandaryl® |
| ACE Inhibitors + Calcium Channel Blocker |
|---|
| PREFERRED AGENTS |
| Lotrel® Tarka® |
| Angiotensin Receptor Blockers |
| Benicar® Cozaar® Diovan® Micardis® |
| Anti-Emetics-Oral |
| PREFERRED AGENTS |
| Kytril®(tablet, solution) Zofran®(tablet, solution, ODT) |
| Beta Blockers |
| PREFERRED AGENTS |
| acebutolol atenolol betaxolol bisoprolol funerate metoprolol tartrate nadolol pindolol propranolol |
| Bisphosphonates-Oral |
| PREFERRED AGENTS |
| Fosamax®(tablet, solution) Fosamax®Plus D |
| Calcium Channel Blockers (DHP) |
| PREFERRED AGENTS |
| Afeditab CR® Dynacirc® Dynacirc CR® felodipine ER isradipine nicardipine Nifediac CC® Nifedical XL® nifedipine Nifedipine ER nifedipine SA Norvasc® Sular® |
| HMG-CoA Reductase Inhibitors (Statins) |
| PREFERRED AGENTS |
| Advicor® Altoprev® Crestor® Lescol® Lescol XL® Lipitor® Vytorin® Zocor® |
| Narcotics-Long Acting |
| Duragesic® fentanyl patch Kadian® morphine sulfate SR Oramorph SR® |
| Sedative Hypnotics/Sleep Agents |
| PREFERRED AGENTS |
| Ambien CR® chloral hydrate estazolam flurazepam temazepam triazolam |
| Steroids-Intranasal |
| PREFERRED AGENTS |
| Nasacort AQ® Nasonex® |
| Triglyceride Lowering Agents |
| PREFERRED AGENTS |
| gemfibrozil Lofibra® |
Attention
Pharmacy Providers
Integrated Voice Response System
for Prior Authorization Programs
Return to Table of Contents
Beginning September 28, 2006, prior authorization validation for all pharmacy prior authorization programs will be processed using an integrated voice response system (IVR).
Certified pharmacy technicians and pharmacists will still be available to answer any questions you have or to assist with technical problems. Below are detailed instructions for using the IVR prior authorization validation system.
Remember, the prescriber must initiate the prior authorization process.
Instructions

Call (877) 309-9493 prior to dispensing to validate all pharmacy prior authorization numbers.
Select Option "2" for Pharmacy.
- A. If the prior authorization number on the prescription ends with the letter "W," select "1".
- B. If the prior authorization number on the prescription does not include the letter "W", select "2".
- C. If you require technical assistance with the prior authorization process, select "3".
When selecting "2" to validate prior authorization numbers that do not end with the letter "W," you will be transferred to the existing VIPS line and will validate the prior authorization number according to current procedures.
Follow these steps after selecting "1" to validate prior authorization numbers that end with the letter "W".
- You will first be prompted to enter the 11-digit prior authorization number found on the face of the prescription. It is not necessary to enter the letter "W" at the end of the number.
- The phone system will retrieve the information for this prior authorization as obtained from the prescriber.
- The phone system will repeat the Client Identification Number (CIN) associated with this prior authorization number and ask you to confirm that this is the correct CIN by pressing "1".
- Once the CIN is confirmed, you will be asked to enter your 8-digit Pharmacy eMedNY Provider Identification Number.
- The phone system repeats this number and asks you to confirm that it is correct by pressing "1". It will then check this number against the NYS Medicaid Pharmacy Provider database to confirm that it is valid.
- Once your Provider ID has been confirmed, you will be asked to enter your Pharmacy Telephone Number.
- The phone system will repeat your telephone number back and ask you to confirm that it is correct by pressing "1".
- Once your Telephone Number has been confirmed, you will be asked to enter the 11-digit NDC number for the product you are dispensing.
- The phone system will repeat the NDC back and ask you to confirm that it is correct by pressing "1". It will then verify that the NDC entered is valid.
- Once the NDC has been confirmed, you will be asked to enter the Quantity per fill and the number of refills from the prescription.
- The phone system will then provide confirmation that you have authorization to dispense the drug.
- You will then be asked if you wish to validate another prior authorization number.
Additional Information
Additional information is available at www.nyhealth.gov or http://newyork.fhsc.com
Questions? Please call (877) 309-9493 and select Option "3" for technical assistance.
Official Prescription Program Update
Return to Table of Contents

Practitioners in hospitals and designated non-profit diagnostic and treatment centers are exempt from the requirement to prescribe non-controlled substances on an official New York State prescription until April 19, 2007. However, all written prescriptions for controlled substances must be issued on an official prescription.
Other than for prescriptions issued from the above-mentioned facilities, the dispensing exemption that allows pharmacists to dispense prescriptions for non-controlled substances that are not written on an official New York State prescription form will expire on October 19, 2006. Therefore, effective October 19, written prescriptions for both controlled and non-controlled substances must be dispensed only pursuant to an official New York State prescription.

Practitioners are required by law to safeguard all their official New York State prescription forms against loss, theft, or unauthorized use. The law also requires practitioners to immediately notify the Official Prescription Program of any such loss, theft, or unauthorized use as well as the failure to receive official prescriptions within a reasonable time after ordering them.
For a notification form or for more information regarding official prescriptions, please contact the Official Prescription Program at:
(866) 811-7957.
Return of Unused Medication from Nursing Facilities
Return to Table of Contents
Nursing facilities and the pharmacies that serve them must ensure that drugs paid for by Medicaid, which are restocked and re-dispensed, are properly credited to the Medicaid Program.
It is against federal law to re-bill Medicaid if you reuse any returned medications that have not been properly credited.
Federal Law Prohibits Double Billing
In accordance with the federal Deficit Reduction Act of 2005, effective April 1, 2006, pharmacies are specifically prohibited from double billing Medicaid for the cost of medications for which the pharmacy has already received payment.
State Regulation Defines Returns and Credit Requirements
The prohibition on double billing is in addition to existing New York State Department of Health regulations (10 NYCRR 415.18 (f)) which require that nursing home facilities have established policies and procedures for the return of unused medication to their vendor pharmacies.

The regulation further requires that vendor pharmacies reimburse or credit Medicaid for unused medication that is restocked and re-dispensed.
Compliance with the regulation is mandatory and is enforced by audit.
This regulation applies to all nursing home facilities regardless of payor and is not solely a Medicaid regulation.
For your information, the New York State regulatory requirements may be accessed on the Department of Health's web site at:
www.nyhealth.gov/regulations/nycrr/title_10/
Claim Reversals and Adjustments/Re-bills
Regarding the correction of billings for drugs returned to the pharmacy, the current Medicaid system is capable of processing re-bill/adjustment transactions, as well as reversal transactions.
If you need assistance with procedures for the correction of billings, please contact Computer Sciences Corporation at:
(800) 343-9000.
Questions? Please contact the Pharmacy Policy & Operations Unit at (518) 486-3209.
Reimbursement for Automated Urinalysis in a
Practitioner's Office
Return to Table of Contents
Effective for dates of service on or after September 1, 2006, Medicaid enrolled physicians, nurse practitioners and midwives will receive Medicaid reimbursement for performing the following tests for their patients in their offices.
To claim reimbursement, practitioners must have a Clinical Laboratory Improvement Amendments (CLIA) certification for waived, moderate or high complexity laboratory testing.
| Code | Description | Fee |
|---|---|---|
| 81001 | Urinalysis, by dip stick or tablet reagent for bilirubin, glucose, hemoglobin, ketones, leukocytes, nitrite, ph, protein, specific gravity, urobilinogen, any number of these constituents; automated, with microscopy (Not payable in combination with 81000). | $ 4.00 |
| 81003 | Urinalysis, by dip stick or tablet reagent for bilirubin, glucose, hemoglobin, ketones, leukocytes, nitrite, ph, protein, specific gravity, urobilinogen, any number of these constituents; automated, without microscopy (Not payable in combination with 81002). | $ 2.00 |
Medicare reimburses for these tests at 100 percent. No Medicare co-insurance payments may be billed to Medicaid for the above listed tests.
For additional information regarding coverage for practitioner office laboratory test billing, please contact the Bureau of Policy Development and Agency Relations staff at (518) 473-2160.
Payment for Speech Generating Devices in Facilities
Return to Table of Contents
Speech generating devices (SGDs) are electronic or non-electronic speech aids that provide an individual who has severe speech impairment with the ability to meet functional speaking needs.

SGDs can be either standard or customized durable medical equipment (DME) and are considered included in the Medicaid per diem rate paid to skilled nursing facilities (SNFs) and developmental centers (DCs).
As outlined in the June 2002 issue of Medicaid Update, standard or customized DME for use by residents of SNFs and DCs will be reimbursed through the per diem rate structure in conformance with Title 10 NYCRR 86-2.22 and the American Hospital Association depreciation guidelines.
Facilities are obligated to directly reimburse the DME vendor for medically necessary SGDs.
If you have any questions regarding Medicaid reimbursement for speech generating devices or other DME in a SNF or DC, please contact the Division of Medical Review and Provider Enrollment at: (518) 474-8161.
Prepayment Review Will End
For Some Procedures
Return to Table of Contents
The Division of Medical Review and Provider Enrollment is eliminating prepayment review on the procedure codes listed below.
Effective for dates of service on and after October 1, 2006, supporting documentation and paper billing will no longer be required as a part of this review.
| G0125-G0336 | All Positron Emission Tomography (PET) scans - including the replacement codes (78459, 78491, 78492, 78608, 78609, 78811, 78812, 78813, 78814, 78815 and 78816; effective 1/1/06). |
| J0150 | Injection, adenosine for therapeutic use 6mg (not to be used to report any adenosine phosphate compounds). |
| J0475 | Baclofen 10 mg. |
| J0585 | Botulinum Toxin Type A, per unit. |
| J0587 | Botulinum Toxin Type B, per 100 units. |
| J9212 | Injection Interferon Alfacon-1 Recombinant 1 mcg. |
| 11043 | Debridement skin, subcutaneous tissue, and muscle. |
| 11044 | Debridement skin, subcutaneous tissue, muscle, and bone. |
| 12001 | Simple repair of superficial wounds of scalp, neck, axillae, external genitalia, trunk and/or extremities (including hands and feet); 2.5 cm or less. |
| 12011 | Simple repair of superficial wounds of face, ears, eyelids, nose, lips and/or mucous membranes; 2.5cm or less. |
All services rendered during the period of September 1, 2005 through September 30, 2006, other than Medicare coinsurance claims, must continue to meet the requirements as originally presented in the August 2005 Medicaid Update.
Those procedure codes included in the August 2005 article and not listed above, will continue to require paper billing and supporting documentation for the prepayment review.
Questions? Please contact the Division of Medical Review and Provider Enrollment Medical Pended Claims Unit at (800) 562-0856.
Krabbe Disease
Payment for Testing and Treatment
Return to Table of Contents
What is Krabbe Disease?
Krabbe Disease is a rare disorder caused by an enzyme deficiency which affects both the central and peripheral nervous systems.
The disease generally presents in the first six months of life and there are usually no obvious congenital anomalies present at birth.
Symptoms
Early symptoms include:
- feeding difficulties,
- gastroesophageal reflux,
- irritability, and
- clasped thumbs,
- followed by rapid physical and mental deterioration.
If not treated early, most patients do not survive past the age of two.
Mandated Newborn Screening and Medicaid Reimbursement
Because of the need for a rapid diagnosis and initiation of therapy for confirmed cases, the New York State Newborn Screening Program began testing for Krabbe Disease in August 2006 as part of the mandated newborn screening panel.
The New York State Medicaid Program covers the testing and treatment of Krabbe Disease, which includes, but is not limited to, the following procedures:
- Newborn screening and confirmatory testing;
- Evaluations and consultations by a metabolic disease specialist and/or child neurologist;
- Neurological testing, including lumbar puncture, MRI, nerve conduction studies, visual evoked response and brain stem auditory evoked response;
- Myeloablative chemotherapy; and
- Newborn and young infant umbilical cord blood transplantation, which, prior to onset of symptoms, has been shown to stabilize the Disease.
Questions about Krabbe Disease screening and treatment protocol can be directed to the NYS Newborn Screening Program at (518) 473-7552.
Questions about NYS Medicaid payment for testing and treatment of Krabbe Disease can be directed to the Division of Medical Review and Provider Enrollment at (518) 474-8161.
New Process for Orthodontic Prior Approval
In Nassau and Suffolk Counties
Effective September 1, 2006
Return to Table of Contents
Effective September 1, 2006, orthodontic cases for Medicaid-eligible recipients up to 21 years of age who are the financial responsibility of Nassau or Suffolk counties will require review and prior approval by the Dental Prior Approval Unit of the New York State Department of Health located in Albany.
Once prior approved, any Medicaid enrolled, board qualified or certified Orthodontist with specialty designator 801 or any clinic facility with specialty designator 912 will be able to provide care to recipients eligible for Orthodontic benefits under the Program on a fee-for-service basis.
Medicaid approval will only be issued for cases presenting with severe handicapping malocclusions.
Procedure Codes Affected
The following procedure codes will now be subject to the Medicaid prior approval process for recipients from Nassau and Suffolk counties:
- D8070 (Comprehensive orthodontic treatment of the transitional dentition);
- D8080 (Comprehensive orthodontic treatment of the adolescent dentition);
- D8090 (Comprehensive orthodontic treatment of the adult dentition-up to age 21);
- D8670 (Periodic orthodontic treatment visit - as part of contract); and
- D8680 (Orthodontic retention).
Patients of the Physically Handicapped Children's Program
The new Medicaid prior approval review process does not apply to Medicaid patients who have a treatment authorization previously issued through the Physically Handicapped Children's Program (PHCP).
Such patients may complete the currently authorized treatment year after which it will be required that a renewal treatment request for prior approval evaluation under Medicaid must be obtained.
For such continuing cases, attach a copy of the previous year's PHCP authorization with the prior approval request.
Prior authorization for non-Medicaid children covered by the Physically Handicapped Children's Program (PHCP) Dental Rehabilitation Program in Nassau and Suffolk counties will change as of September 1, as well:
- These children will no longer have to attend screening clinics.
- Prior authorization requests for PHCP coverage will be transferred from the screening clinics to the Bureau of Dental Health in Albany.
Prior authorization requests for PHCP-enrolled children and required documentation (DOH-4268, treatment plan, intra-oral photographs, cephalometric X-ray and panoramic X-ray) should be submitted to the following address:
Bureau of Dental Health
Room 542, Corning Tower
Empire State Plaza
Albany, New York 12237-0619
General dental conditions, including restorations, prophylaxis and extractions should be addressed by a general dentist prior to any request for orthodontics being submitted, whether for Medicaid-enrolled or PHCP-enrolled children.
Prior Approval Request Forms
Prior Approval request forms completed pursuant to instructions contained in a recent letter to orthodontists in Nassau and Suffolk counties and in the Medicaid Dental Provider Manual, along with appropriate diagnostic aids and supporting information should be sent to:
P.O. Box 4600
Rensselaer, New York 12144-4600
Specialty Designation Forms
Orthodontists wishing to obtain the appropriate specialty designation can obtain the necessary form at:
http://www.emedny.org/info/ProviderEnrollment/index.html or
contact Computer Sciences Corporation at: (800) 343-9000, option 5.
Questions?
Questions regarding this new Medicaid prior approval requirement should be directed to the Division of Medical Review and Provider Enrollment, Dental Prior Approval Unit at:
(800) 342-3005, option 2.
Questions regarding the new PHCP prior authorization requirement should be directed to the Bureau of Dental Health at:
(518) 474-1961.
Clarification of Medicaid Coverage
For Physical Examinations and Travel Immunizations
Return to Table of Contents
The purpose of this article is to provide policy guidance on Medicaid coverage for physical exams and travel immunizations.
Physical Examinations

The Medicaid Program only covers services that are medically necessary.
Physical examinations that are considered to be medically necessary include:
- School physicals;
- Camp physicals; and
- Physical or mental health examinations of children and their parents as requested by the Local Social Services District for the protection of adults and children in foster care.
The following types of physical exams are not covered:
- Physical examinations required by employers for employment purposes.
It is the employer's responsibility to pay for the physical examination as cited in the New York State Labor Law, Article 7, Section 201-b. - Physical examinations or mental health assessments for the purpose of making recommendations regarding a recipient's disability status for Federal SSI applications.
- Physical examinations required by the Local Social Services District as a consultative exam for Medicaid disability review purposes.
It is an administrative cost to the Local Social Services District. - Physical examinations required by the Local Social Services District to determine employability of public assistance recipients.
It is an administrative cost to the Local Social Services District.

Travel Immunizations
Immunizations required for travel in or outside the United States may be considered medically necessary and may therefore be reimbursable under the Medicaid Program.
Questions? Please contact the Bureau of Policy Development and Agency Relations at (518) 473-2160.
Proper Billing Requirements for Clinics
Procedure Codes
Return to Table of Contents

Procedure Codes
When billing eMedNY for services provided by a hospital-based or freestanding clinic, providers are required to include the appropriate HCPCS procedure code(s) that identifies the service(s) rendered to a recipient.
The procedure code entered on the claim must reflect the actual service rendered to the patient.
Appropriate procedure codes should be used when multiple services are rendered in the same clinic visit:
- for HIPAA 837 (Institutional) claims, the procedure code must be reported in Loop 2400, SV Segment; and
- neither the rate code (nor any other non-procedure code) should be entered in the procedure code field.
The procedure code reported must be consistent with the scope of practice, certification and/or profession of the rendering provider.
For example, an Evaluation and Management code may only be reported on a clinic claim when the service is rendered by a qualified licensed practitioner, such as a physician, nurse practitioner, licensed midwife or physician assistant.
Note: Dental clinics should enter the five-character CDT-4 dental procedure code (subsumed into HCPCS for HIPAA).
Diagnosis Codes
Additionally, clinics are required to include an appropriate diagnosis code which reflects the condition being treated at the clinic visit.
The principal diagnosis (primary reason for the visit) should be reflected in the ICD-9 CM diagnosis code that is reported on the claim.
Applicability
These requirements apply to all clinics whether certified by the Department of Health, the Office of Mental Health, the Office of Alcoholism and Substance Abuse Services, or the Office of Mental Retardation and Developmental Disabilities.
The Medicaid Program pays for medically necessary medical care. Accurate procedure and diagnosis coding helps us to ensure Medicaid recipients are getting high quality, appropriate services.
Care must be taken to follow the coding instructions carefully. The Department edits claims for diagnosis codes, and plans to begin editing claims for accurate procedure codes. Coding errors could result in denied claims.
In an effort to avoid the costs associated with denied claims, providers should take this opportunity to verify procedure codes before the edits are set to deny claims.
Questions? Please contact the Bureau of Policy Development and Agency Relations at (518) 473-2160.
Attention
- Office of Mental Retardation and Developmental Disabilities (OMRDD) Voluntary Agencies Operating Intermediate Care Facilities for the Developmentally Disabled (ICF/DD).
- OMRDD Article 16 Clinic Providers,
- Department of Health (DOH) Article 28 Clinic Providers, and
- Clinical Practitioners
Certain Long Term Therapy Services To Be Incorporated Into Intermediate Care Facilities
For The Developmentally Disabled Medicaid Payment Rates
Effective January 1, 2007
Return to Table of Contents
Starting with services rendered January 1, 2007, each Office of Mental Retardation and Developmental Disabilities (OMRDD) certified Intermediate Care Facility for the Developmentally Disabled (ICF/DD) is fiscally responsible for the long term therapies identified below:

- occupational therapy,
- physical therapy,
- psychologist services,
- speech and language pathology,
- social work,
- dietetics and nutrition,
- rehabilitation counseling, and
- nursing services (excluding medical services provided by a nurse practitioner).
Separate Medicaid billing of these long term therapies by clinical practitioners, Article 16, and Article 28 clinics will be prohibited when provided to an ICF/DD resident, regardless of the service location.
Separate time-limited billing of specified therapies will be allowed in response to acute illness, an accident, or a post-hospitalization health need.
OMRDD sent letters to all certified ICFs/DD on May 19, 2006 and June 13, 2006 that contain details about this exception. (Copies of these letters were also sent to OMRDD Article 16 clinics.)
Note: Some of the services listed above are never billable by Article 28 certified clinics.
For ICFs/DD that do not have day treatment program funding included in their rate, separate billing of day treatment to Medicaid continues to be allowed.
Questions? Please contact Karen Desso of OMRDD at (518) 402-4339.
Coverage of Specialist Consultations
via Telemedicine
Return to Table of Contents
Effective for dates of service on and after September 1, 2006, medically necessary emergency room and inpatient hospital consultation services are payable to physicians with a specialty designation providing consultations via an interactive audio and video telecommunication system.
What is Telemedicine?
An interactive audio and video telecommunications (telemedicine) system i is a type of technology that permits a "real time" interactive consultation service to take place between the physician and patient.
Telemedicine is reimbursable when a patient is located at a spoke site and the needed specialist is located at a hub site.
- The hub site is where the medical specialist is located (e.g., hospital, office).
- The spoke site is the hospital where the referring health professional and patient are located.
When is Telemedicine Covered?
- A consultation involving a present and participating patient and a specialist is medically necessary, and a specialist is not available at the spoke site to provide a timely consultation;
- The telemedicine system used for the consultation is a fully interactive, secure two-way audio and video telecommunication system and also supports review of diagnostic tests integral to the consultation;
- A request for a consultation and the need for a consultation is documented in the patient's medical record;
- The consultation opinion is documented in the patient's medical record and communicated to the requesting provider;
- The consultation code is billed with the appropriate modifier "-GT via interactive audio and video telecommunication systems" to indicate services were performed via telemedicine;
- The consulting physician is licensed in New York State, practicing within the scope of his/her specialty practice, enrolled in the New York State Medicaid Program and meets the credentialing requirements of the spoke site hospital.
Physician Billing for Telemedicine
- Payment for telemedicine specialist consultations will be limited to codes 99241-99245 and 99251-99255. Reimbursement will be the same amount as in-person specialist consultations;
- The specialist at the hub site bills the consult code with the -GT modifier;
- The emergency room or attending inpatient physician at the spoke site bills the applicable evaluation and management code without the -GT modifier, (Note: if evaluation and management services are already included in the emergency room or inpatient rate then the respective physician cannot bill an evaluation and management code);
- Payment will be made to only one physician for the professional component (reading and interpretation) of diagnostic tests such as radiological procedures and diagnostic assessments;
- If specialist services are included in the facility rate where the patient is admitted, no separate consultant physician payment is reimbursable;
- The place of service entered on the claim is the location of the patient: "21" for inpatient hospital and "23" for emergency room-hospital.
- If the telemedicine consultation service is owned by a hub hospital and relevant specialist services are already included in the hub facility's rate, then no separate reimbursement is permissible for telemedicine consultations performed by employed specialists.
More Information

For information on funding for rural hospitals for purchasing of telemedicine equipment, please contact the Office of Rural Health at:
(518) 474-5565.
For information on credentialing requirements, please contact the Office of Health Systems Management at:
(518) 408-1828.
For information on procedure codes or fees, please refer to the Physician Fee Schedule, available at:
http://www.emedny.org/ProviderManuals/Physician/PDFS/Physician_Fee_Schedule_2006.pdf, or
contact the Division of Medical Review and Provider Enrollment at (518) 474-8161.
For information on claim form completion, please contact Computer Sciences Corporation at:
(800) 343-9000.
General inquiries may be sent via email to: telemed@health.state.ny.us.

Medicaid statistics, including the number of monthly Medicaid eligibles and expenditure reports statewide and/or by county, are available online at.
http://www.nyhealth.gov/nysdoh/medstat/medicaid.htm
Annual Recertification for
Providers Submitting Electronic Claims
Return to Table of Contents
Providers who submit electronic claims to the New York State Medicaid Program are required by the Department to submit a signed and notarized Certification Statement on a yearly basis.
Signing the Certification Statement binds a provider to the requirements put forth in the Certification Statement.

Providers need to read and understand the Certification Statement requirements before signing.
The certification process links the provider's assigned Medicaid provider identification number to the Electronic Transmitter Identification Number (ETIN) under which electronic claims are submitted.
The Certification Statement is kept on file and may be presented by the State's Attorney General's office when prosecuting providers for fraudulent billing practices.
Certification Statements remain in effect and apply to all claims until superseded by another properly executed Certification Statement. You will be asked to update your Certification Statement annually.
If you receive a recertification notice, your time to recertify is nearing.
Providers are sent two notices to recertify, each containing the date your current certification will expire.
Failure to recertify will cause your claims to be rejected beginning with the decertification date in the notices.
When you receive a recertification notice, please read the Certification Statement carefully, complete the form, including notarization, and submit it to the following address:
Enrollment Support
1 CSC Way
Rensselaer, New York 12144
Questions? Please contact Computer Sciences Corporation at (800) 343-9000 option 5.
Prevent Disease: Immunize Please!
Who Needs Immunizations?
Return to Table of Contents
Who Needs Immunizations?
Most infants, toddlers, school-age children and college students are required to have certain immunizations. But, immunizations are not just for kids and students. Adults, too, need to be protected against such preventable diseases as measles, mumps, rubella, tetanus, diphtheria, pneumococcal disease, influenza, hepatitis B and others.
The best protection against these diseases is immunization.
How Often Should a Child be Immunized?
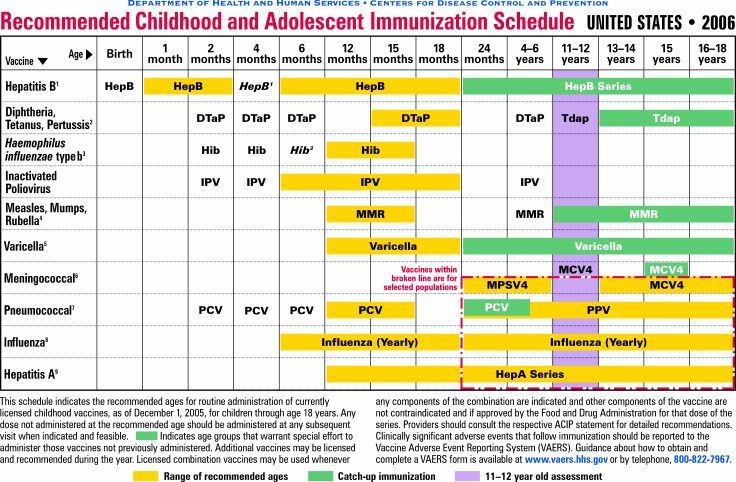
How Often Should an Adult be Immunized?
The following chart indicates the Centers for Disease Control and Prevention's recommended vaccinations by age group:
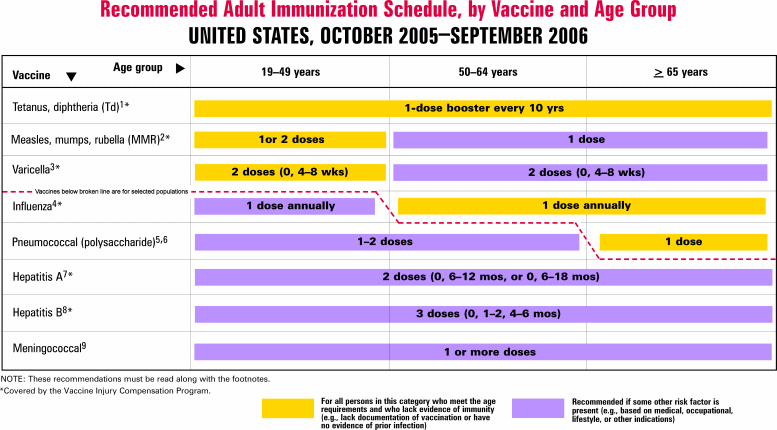
More Information
For more information on immunizations and the complete adult and childhood immunization schedules, consult with your primary physician or go to the Department of Health's website:
http://www.nyhealth.gov/prevention/immunization/index.htm.
Questions? Please contact the Program Quality and Initiatives Unit at (518) 474-9219.
Long Term Home Health Care Program
Reference Manual
Return to Table of Contents
The Long Term Home Health Care Program (LTHHCP) Reference Manual is now available online at:
http://www.health.state.ny.us/health_care/medicaid/reference/lthhcp/
.The Manual is a useful tool for providers which consolidates and clarifies information related to the LTHHCP, and can be printed from the website above.
The Manual:

- explains the LTHHCP,
- provides Program rules and guidelines,
- addresses the assessment process,
- defines and clarifies various services included in the Program, and
- clarifies the monthly budgeting process and includes several case studies and examples.
It also contains:
- a glossary,
- DOH approved LTHHCP forms, and
- a listing of policy directives pertaining to the Program.
Questions? Please contact the Bureau of Long Term Care at (518) 474-6580.
Do you suspect that a recipient or a provider has engaged in fraudulent activities?
Please call:
1-877-87FRAUD
(1-877-873-7283)
Your call will remain confidential.
Attention
All Providers
Medicaid Requires Providers to Stay Informed of Policy and
Claiming Rules
Return to Table of Contents

Your acceptance as a New York State Medicaid provider requires that you accept the policies and regulations of the Medicaid Program and directives of the New York State Department of Health, which include, but are not limited to, Part 504 of Title 18 of the New York Code of Rules and Regulations, available online at:
http://www.nyhealth.gov/regulations/nycrr/title_18/
You are responsible to ensure your compliance with the policies and regulations of the Medicaid Program.
This information is available in:
- pertinent information mailed via the monthly Medicaid Update; and,
- your Provider Manual.
All changes in policy are included in the Medicaid Update. Subsequently, this information will appear in an updated version of your Provider Manual.
The Medicaid Update
The Medicaid Update is a monthly newsletter from the New York State Medicaid Program that announces major policy changes and other important Medicaid-related information. The Medicaid Update is mailed to enrolled providers who submit claims to Medicaid.
The Medicaid Update is mailed to the correspondence address you gave to the Medicaid Program as part of your enrollment. If this address changes, you should complete and submit the appropriate Address Change Form.
Address Change Forms are available online at:
http://www.emedny.org/info/ProviderEnrollment/index.html
or by calling Computer Sciences Corporation at (800) 343-9000, option 5.
If you would like to receive the Medicaid Update electronically, please email your request to:
MedicaidUpdate@health.state.ny.us
The Medicaid Update is also available online at:
http://www.nyhealth.gov/health_care/medicaid/program/update/main.htm
Provider Manual
Your Provider Manual is specific to your provider type, and contains in-depth Medicaid policy information, Medicaid resources, how to claim for services rendered, fee schedules, and additional information crucial to maintaining compliance with Medicaid rules and regulations.
Your complete Provider Manual can be downloaded and/or printed from the eMedNY website at:
http://www.emedny.org/ProviderManuals/index.html
As a provider, it is your responsibility to check this website on a monthly basis to ensure you are current with the latest policy information.
If you do not have access to the internet, you must contact Computer Sciences Corporation (CSC) to receive a hard copy of your provider manual at:
(800) 343-9000.
You must call CSC monthly and request a hard copy of changes made since your previous request.
Attention
Rate-Based
Institutional
Providers
Disclosure of Ownership Document
Return to Table of Contents
It is the responsibility of each provider to notify the New York Medicaid Program of any changes to the information which was supplied at the time of enrollment.
This includes changes for organizations or individuals having direct or indirect ownership or a controlling interest of five-percent or more in the enrolled agency, institution or organization.
If this information has changed since the time of enrollment, you are required to complete and submit a new Disclosure of Ownership document to the Medicaid Program so that your Provider file may be updated.
Currently, the Form may be obtained by calling the Office of Medicaid Management's Rate Based Provider Unit at: (518) 474-8161 or you can e-mail a request to RBU@health.state.ny.us.
The request should state "Request Disclosure Form" in the attention/subject line and contain the provider identification number and name of the entity. A Form will be emailed to you.
Upon completion of the Form, please return via postal mail to:
Office of Medicaid Management
Division of Medical Review and Provider Enrollment
Rate Based Provider Unit
150 Broadway
Albany, New York 12204-2719
This Form will be available on the http://www.eMedNY.org web site in the future.
Citizenship Documentation Requirements of
The Deficit Reduction Act of 2005
Effective July 1, 2006
Return to Table of Contents

The Deficit Reduction Act of 2005 (DRA) amends federal Medicaid statute to require that all United States citizens applying for or renewing their Medicaid coverage provide "satisfactory documentary evidence" of their citizenship. There has been much misinformation about these provisions circulating in the community. This article explains the provisions of the DRA and their impact on Medicaid applicants/recipients in New York State.
Documentation of citizenship status is not a new requirement for the New York State Medicaid Program.
Because New York State currently requires documentation of citizenship, the Department anticipates the new rules will have minimal impact on New York State Medicaid applicants/recipients.
Pregnant women will continue to be eligible for perinatal care in New York without regard to citizenship of immigration status.
The DRA establishes acceptable documentation for citizenship and identity. The following are defined as primary documents of citizenship and identity:
- A U.S. passport, and
- A Certificate of Naturalization or a Certificate of U.S. Citizenship.
A birth certificate is still acceptable as proof of citizenship, but can no longer be used to prove identity.
If you are a provider who assists individuals in applying for Medicaid, you should be aware that when a birth certificate is presented as proof of citizenship, another form of identity document, such as a driver's license, must accompany the submitted application.
The DRA requirement applies to U.S. citizens and those individuals who allege to be U.S. citizens. The documentation requirements do not affect immigrant applicants for Medicaid. New York provides Medicaid to qualified immigrants and, unlike some other states, covers qualified immigrants in the five-year ban and persons permanently residing in the United States under the Color of Law (PRUCOL) with State funds. The State requires documentation of satisfactory immigration status from those applicants who are immigrants.
Medicaid policy for the coverage of immigrants has not changed, nor is it affected by the DRA.
The Department is currently reviewing these provisions to determine if any of our citizenship documentation requirements need to be amended.
Further instructions will be provided as necessary in subsequent issues of the Medicaid Update.
Additional information may be obtained on the Centers for Medicare and Medicaid Services website:
Questions? Please call (518) 473-5330.
New York State Medicaid Online
Return to Table of Contents

Would you like to learn more about the New York State Medicaid Program?
Visit any of the websites below for more information.
| Medicaid Program | http://www.nyhealth.gov/health_care/medicaid/ |
| eMedNY | http://www.emedny.org/ |
| EPIC for Seniors | http://www.nyhealth.gov/health_care/epic/index.htm |
| Formulary File | http://www.emedny.org/info/formfile.html |
| Health Insurance Programs | http://www.nyhealth.gov/health_care/ |
| Local Departments of Social Services | http://www.ocfs.state.ny.us/main/localdss.asp#r |
| Medicaid Managed Care | http://www.nyhealth.gov/health_care/managed_care/index.htm |
| Medicaid Update | http://www.nyhealth.gov/health_care/medicaid/program/update/main.htm |
| Medicaid Statistics | http://www.nyhealth.gov/nysdoh/medstat/medicaid.htm |
| Medicare | www.medicare.gov |
| Official Prescription Program | http://www.nyhealth.gov/professionals/narcotic/index.htm |
| Provider Enrollment | http://www.emedny.org/info/ProviderEnrollment/index.html |
| Provider Manuals | http://www.emedny.org/ProviderManuals/index.html |
For questions about the New York State Medicaid Program, please send an email to:
MedicaidUpdate@health.state.ny.us
Your question will be answered as soon as possible and/or forwarded to the appropriate party.
Would You Like to Receive
the Medicaid Update Electronically?
Return to Table of Contents
To request the electronic version, send an email to the Medicaid Update at:
MedicaidUpdate@health.state.ny.us
Please provide the following information:
- Your Name
- Medicaid Provider ID Number
- Email address (or multiple addresses, if desired).

PROVIDER SERVICES
Return to Table of Contents
Missing Issues?
The Medicaid Update, now indexed by subject area, can be accessed online at the New York State Department of Health website:
http://www.nyhealth.gov/medicaid/program/update/main.htm
Hard copies can be obtained upon request by emailing: MedicaidUpdate@health.state.ny.us
Do You Suspect Fraud?
If you suspect that a recipient or a provider has engaged in fraudulent activities, please call the fraud hotline at: 1-877-87FRAUD. Your call will remain confidential.
As a Pharmacist, Where Can I Access the List of Medicaid Reimbursable Drugs?
The list of Medicaid reimbursable drugs is available at: http://www.eMedNY.org/info/formfile.html
Questions About an Article?
For your convenience each article contains a contact number for further information, questions or comments.
Do You Want Information On Patient Educational Tools and Medicaid's Disease Management Initiatives?
Contact Department staff at (518) 474-9219.
Questions About HIPAA?
Please contact CSC Provider Services at (800) 343-9000.
Patient Eligibility
Call the Touchtone Telephone Verification System (800) 997-1111, (800) 225-3040 or (800) 343-9000.
Address Change?
Questions should be directed to CSC at (800) 343-900, option 5.
Fee-for-service Provider Enrollment
A change of address form is available at:
http://www.emedny.org/info/ProviderEnrollment/Provider%20Maintenance%20Forms/6101-Address%20Change%20Form.pdf.
Rate-based/Institutional Provider Enrollment
A change of address form is available at:
http://www.emedny.org/info/ProviderEnrollment/Provider%20Maintenance%20Forms/6106-Rate%20Based%20Change%20of%20Address%20Form.pdf
Billing Question? Call Computer Sciences Corporation:
Provider Services (800) 343-9000.
Comments and Suggestions Regarding This Publication?
Please contact the editor, Timothy Perry-Coon at MedicaidUpdate@health.state.ny.us or via telephone at (518) 474-9219 with your concerns.
The Medicaid Update: Your Window Into The Medicaid Program
The State Department of Health welcomes your comments or suggestions regarding the Medicaid Update.
Please send suggestions to the editor, Timothy Perry-Coon:
NYS Department of Health
Office of Medicaid Management
Bureau of Program Guidance
99 Washington Ave., Suite 720
Albany, NY 12210
(e-mail MedicaidUpdate@health.state.ny.us)
The Medicaid Update, along with past issues of the Medicaid Update, can be accessed online at the New York State Department of Health web site:http://www.health_care/medicaid/program/update/main.htm