New York State Medicaid Update - June 2016 Volume 32 - Number 6
In this issue …
- All Providers
- Behavioral Health Transition to Managed Care (Outside of New York City) – What Providers Need to Know
- Mandatory Compliance Program Certification Requirement under 18 NYCRR §521.3(b) - Reminder
- Certification of Compliance with Section 6032 of the Deficit Reduction Act of 2005, Section 1902 of the Social Security Act, and Title 42 of the United States Code Section 1396a(a)(68) - Reminder
- New York Medicaid Electronic Health Record (EHR) Incentive Program Update
- How can the Women, Infants and Children (WIC) program help your Clientele?
- New York Medicaid Management Information System (NYMMIS) Continuous Improvements to Training
- Policy and Billing Guidance
- Incontinence Supply Management Program September 1, 2016
- New Law in Relation to the Consumer Directed Personal Assistance Program (CDPAP) - Clarification
- Patient Centered Medical Home Statewide Incentive Payment Program - Reminder
- Guidance for New York State Medicaid Enrolled Laboratories Performing Testing for BRCA Mutations
- Pharmacy Update
- Provider Directory
Behavioral Health Transition to Managed Care (Outside of New York City)
What Providers Need to Know
Beginning on July 1, 2016, most behavioral health benefits for adults that were previously paid on a Fee-For-Service (FFS) basis will be "carved-in" to Medicaid Managed Care (MMC) for eligible enrollees outside of New York City. These behavioral health services were carved in for plans and eligible enrollees in New York City on October 1, 2015. Providers are encouraged to refer to the July 2015 Special Edition Medicaid Update to learn more about expansion of behavioral health services in MMC and the services available through a Health and Recovery Plan (HARP).
In addition to providing coverage for most behavioral health benefits for adults, most MMC plans are offering a HARP. HARP is a new MMC plan that includes all of the behavioral health benefits carved in to MMC and is specifically designed to help coordinate physical and behavioral healthcare. These plans are available to Medicaid enrollees with Serious Mental Illness (SMI) and Substance Use Disorders (SUD).
MMC plans have sent announcement letters that explain the changes to the Medicaid program. Additionally, the State's enrollment broker, New York Medicaid Choice, began sending enrollment notices to HARP-eligible MMC enrollees. These notices will continue to be mailed through June, 2016.
Eligible MMC enrollees will either be passively enrolled or given an option to enroll in a HARP, depending upon current plan enrollment, as follows:
- HARP-eligible enrollees in a MMC plan operated by a Managed Care Organization (MCO) that also operates a HARP product may be passively enrolled into the HARP. The enrollment notice sent by New York Medicaid Choice indicates HARP eligibility, the effective date of HARP enrollment and instructions on how to opt out of enrollment in the Managed Care Organization's HARP.
- HARP-eligible enrollees in MMC plans operated by a MCO not offering a HARP product may actively select and enroll in another MCO's HARP. The notice sent by New York Medicaid Choice indicates HARP eligibility, instructions for enrollees interested in HARP enrollment and instructions on how to obtain information regarding appropriate enrollment options.
It is important for all providers, including primary care providers, to understand the behavioral health transition process since consumers may request assistance in understanding enrollment notices. Providers should also be familiar with their current managed care network affiliations to better assist enrollees interested in HARP selection and to maintain current patient relationships. Additionally, behavioral health providers should ensure that they are contracted with MMC plans, in order to ensure that there is no disruption of payment for these behavioral health services. For additional information on HARP plans, refer to the September 2015 Medicaid Update.
Pharmacy Changes
In accordance with this transition, MMC Plans serving New York State enrollees outside of New York City will also begin covering injectable atypical (second generation) long acting antipsychotics for their Supplemental Security Income (SSI) and SSI related enrollees, and naltrexone extended release suspension (Vivitrol®). Additionally, policies that promote access to these medications and smoking cessation agents are being implemented. The following summarizes these changes.
- Long-Acting Injectable Antipsychotics, typical and atypical
- MMC plans will begin covering atypical injectables for SSI and SSI-related enrollees. Previously, these were covered under FFS.
- MMC plans will cover typical and atypical long-acting injectables as both a pharmacy and medical benefit. Billing and policy guidance will be communicated to providers by the MMC plans.
- Prior authorization (PA) for typical long-acting antipsychotics (e.g., haloperidol decanoate and fluphenazine decanoate) will not be required.
- Medications used for the treatment of Substance Use Disorders (SUD)
- Extended-release naltrexone injectable (Vivitrol®) will be covered as a pharmacy and medical benefit (previously a FFS benefit). Billing and policy guidance will be communicated to providers by the MMC plans.
- MMC plans will include medications for the treatment of SUD and/or opioid dependency on their formularies (not solely through a medical exception process).
- At least one formulation of buprenorphine and buprenorphine/naloxone will be included in plan formularies.
- Clinical criteria for buprenorphine shall also consider lengths of therapy for enrollees transitioning from long acting opioids or who are pregnant or breast feeding.
- Naloxone vials/prefilled syringes and/or the auto-injector and/or atomizer will be covered as a medical and pharmacy benefit.
- The chart on the following page of this article provides guidance for determining coverage (MMC vs. FFS) for risperidone microspheres (Risperdal® Consta®), paliperidone palmitate (Invega Sustenna®, Invega Trinza®), olanzapine (Zyprexa® Relprevv™), aripiprazole (Abilify Maintena®, Aristada®) and naltrexone (Vivitrol®) as of 10/1/2015 & 7/1/2016 respectively.
Member Qualified for SSI or is SSI Related Member Geographic Location Age Coverage Provided By Available through the Medical Benefit Available through the Pharmacy Benefit No Entire State All Ages MMC Plan
(effective 10/1/2011)Yes Yes Yes New York City 21 or older MMC Plan
(effective 10/1/15)Yes Yes Yes Entire State 20 or younger Medicaid FFS Yes No* Yes Outside of New York City 21 or older MMC Plan
(effective 7/1/16)Yes Yes *The Department is working on implementing system changes to allow for atypical long-acting injectables, as well as injectable naltrexone extended release (Vivitrol(®)) to be covered as a pharmacy benefit for those enrollees in MMC who continue to access these medications through Medicaid FFS (as shown above). More information will be communicated on this as we continue to make progress.
- Medications Used For Smoking Cessation
- Course limitations will not apply to enrollees with a SUD and/or a diagnosis of mental illness;
- MMC plans will allow for concomitant utilization of two (2) agents, defined as: two (2) Nicotine Replacement Therapies (NRT); a NRT and bupropion Sustained Release (SR); or a NRT and Chantix.
- Formulary coverage of all smoking cessation agents.
The New York State Medicaid Managed Care Pharmacy Benefit Information Center has been updated to include therapeutic classes affected by the behavioral health transition. This site also provides direct links to MMC web sites, MMC plan contact information, a "drug look-up" option, and functionality to view coverage for selected therapeutic classes; e.g., atypical antipsychotics, smoking cessation agents, etc.
Regional Planning Consortium (RPC) Kickoff Events
The New York State Offices of Mental Health, as well as Alcohol and Substance Abuse Services, and the Department of Health have partnered with the Conference of Local Mental Hygiene Directors to develop RPCs as part of the rest of the State's Medicaid Behavioral Health transition into Managed Care. The RPCs will be a vehicle for key stakeholders, such as consumers, providers, and MMC plans to work together to ensure that the behavioral health transition becomes a positive force for the residents of New York State. The RPCs will provide a local voice to the statewide system transformation.
RPCs will collaborate regionally to ensure such things as timely access to care, efficient claims and payment, and to serve as an early warning for issues that may arise. Kickoff events are now taking place across the State to provide an introduction to the MMC transition and structure of the RPCs. More information about RPCs can be found at http://www.clmhd.org/rpc/.
Mandatory Compliance Program Certification Requirement under Title 18 of the New York Codes, Rules and Regulations (NYCRR) §521.3(b) - Reminder
This is a reminder from the New York State Office of the Medicaid Inspector General (OMIG) for all required providers who are subject to the New York State Social Services Law (SSL) Section 363-d Mandatory Compliance Program Requirement.
On December 1, 2016, OMIG will make available on its website, the New York State Social Services Law Compliance Program Certification Form (Certification Form) for 2016. The Certification Form for 2015 will remain active on OMIG's website until December 1, 2016 for newly enrolling and revalidating Medicaid providers.
OMIG will host a webinar in November 2016 that will explain the new certification form. Please check OMIG's listserv, Facebook page or Twitter feeds for registration information. You can subscribe to OMIG's listserv at www.OMIG.ny.gov.
The required providers listed below must have compliance programs. If you are required to have a compliance program, you are also required to certify on OMIG's web site at www.OMIG.ny.gov, that your compliance program meets the requirements of the applicable law and regulations. The certification must occur in December of each year.
OMIG has actively enforced Social Services Law §363-d and Part 521, of Title 18 of the New York State Codes, Rules and Regulations since 2009. The regulation mandates all required providers under the Medicaid program in the following categories to certify in December of each year that they have adopted, implemented, and maintain an effective compliance program:
- persons subject to the provisions of articles 28 or 36 of the New York State Public Health Law;
- persons subject to the provisions of articles 16 or 31 of the New York State Mental Hygiene Law;
- other persons, providers or affiliates who provide care, services or supplies under the Medicaid program, or persons who submit claims for care, services or supplies for or on behalf of another person or provider for which the Medicaid program is or should be reasonably expected by a provider to be a substantial portion of their business operations.
Under 18 NYCRR §521.2 (b), "substantial portion" of business operations means any of the following:
- (1) when a person, provider or affiliate claims or orders, or has claimed or has ordered, or should be reasonably expected to claim or order at least $500,000 in any consecutive 12-month period from the Medical Assistance Program;
- (2) when a person, provider or affiliate receives or has received, or should be reasonably expected to receive at least $500,000 in any consecutive 12-month period directly or indirectly from the Medical Assistance Program; or
- (3) when a person, provider or affiliate who submits or has submitted claims for care, services, or supplies to the Medical Assistance Program on behalf of another person or persons in the aggregate of at least $500,000 in any consecutive 12-month period.
Each compliance program must contain the eight elements required under SSL §363-d and 18 NYCRR §521.3 (c). Upon applying for enrollment in the medical assistance program, and during the month of December each year thereafter, 18 NYCRR §521.3 (b) requires those subject to the mandatory compliance program obligation to certify to the New York State Department of Health (DOH) and OMIG that a compliance program meeting the requirements of the regulation is in place. For those Medicaid providers required to have a compliance program and to certify in December 2016, OMIG recommends that providers test the operation of their compliance program and make any adjustments necessary so that in December, the Medicaid provider is prepared to certify that its compliance program meets the requirements of SSL §363-d and 18 NYCRR §521.3 (c).
Please note that DOH is revalidating Medicaid providers' enrollment in the medical assistance program. As part of DOH's revalidation process, required providers will be asked to submit evidence that they met the December certification obligation. Certifying in December and retaining a copy of the Certification Confirmation and/or confirmation emails will help Medicaid required providers complete the revalidation process.
The regulation and Frequently Asked Questions (FAQs) are available on the OMIG website. OMIG's listserv subscribers will be notified when the new forms are posted.
It is the responsibility of a required provider to determine if:
- they have a compliance plan that meets the requirements of SSL § 363-d and 18 NYCRR § 521.3 (c); and
- the compliance program is effective.
Required providers must assess their compliance programs to determine whether they can certify that they do or do not have a compliance program in place that meets the requirements of SSL § 363-d and 18 NYCRR Part 521.
Additionally, OMIG recommends a regular visit to its website to review the information and resources that are published under the Compliance tab on OMIG's homepage. The Compliance Library under the Compliance tab provides copies of current forms, publications and other resources that are helpful in conducting a self-assessment and completing the certification form in December.
If you have any questions, please contact OMIG's Bureau of Compliance at(518) 408–0401 or by using the Bureau of Compliance's dedicated email address compliance@omig.ny.gov.
Certification of Compliance with Section 6032 of the Deficit Reduction Act of 2005, Section 1902 of the Social Security Act, and Title 42 of the United States Code Section 1396a (a)(68) - Reminder
This is a reminder from the New York State Office of the Medicaid Inspector General (OMIG) for all providers who are subject to the requirements under Title 42 of the United States Code (USC) Section 1396a (a)(68), [42 Usc §1396a (a)(68)].
On December 1, 2016, OMIG will make available on its website, the federal Deficit Reduction Act (DRA) of 2005 DRA Certification Form (Certification Form) for 2016.
OMIG will host a webinar in November 2016 to explain the new certification form. Please check OMIG's listserv, Facebook page or Twitter feeds for when registration for this session will be available.
42 Usc §1396a provides in relevant part that:
(a) A State plan for medical assistance must—
(68) provide that any entity that receives or makes annual payments under the State plan of at least $5,000,000, as a condition of receiving such payments, shall—
(A) establish written policies for all employees of the entity (including management), and of any contractor or agent of the entity, that provide detailed information about the False Claims Act established under sections 3729 through 3733 of title 31, United States Code, administrative remedies for false claims and statements established under chapter 38 of title 31, United States Code, any State laws pertaining to civil or criminal penalties for false claims and statements, and whistleblower protections under such laws, with respect to the role of such laws in preventing and detecting fraud, waste, and abuse in federal healthcare programs (as defined in section 1320a-7b(f) of this title);
(B) include as part of such written policies, detailed provisions regarding the entity's policies and procedures for detecting and preventing fraud, waste, and abuse; and
(C) include in any employee handbook for the entity, a specific discussion of the laws described in subparagraph (A), the rights of employees to be protected as whistleblowers, and the entity's policies and procedures for detecting and preventing fraud, waste, and abuse.
OMIG addresses this mandate by monitoring a provider's certification of compliance status and conducting compliance program reviews of required providers.
The certification form and Frequently Asked Questions (FAQs) will be available on the OMIG website. OMIG's listserv subscribers will be notified when the new forms are posted.
If you have any questions, please contact OMIG's Bureau of Compliance at(518) 408–0401 or by the dedicated email address compliance@omig.ny.gov.
New York Medicaid Electronic Health Record (EHR) Incentive Program Update
The New York Medicaid EHR Incentive Program provides financial incentives to eligible professionals and hospitals to promote the transition to EHRs. Providers who practice using EHRs are in the forefront of improving quality, reducing costs and addressing health disparities. Since December 2011 over $779 million in incentive funds have been distributed within 24,105 payments to New York State Medicaid providers.
24,105 Payments
$779+ Million Paid
Are you eligible? For more information, visit www.emedny.org/meipass
MEIPASS Availability
New York Medicaid's attestation system (MEIPASS) will not be available starting July 1, 2016. The system will be undergoing important maintenance for Meaningful Use attestations for 2015 and beyond. During this maintenance period, users will not be able to access MEIPASS.
It is anticipated that MEIPASS will reopen during the third quarter of calendar year 2016. Announcements will be made via LISTSERV and the program website.
Program support will continue to be available by phone at 877-646-5410 Option 2 and by email at hit@health.ny.gov.
Pre-Validation Services
Eligible professionals (EPs) who have already determined their Medicaid patient volume may request pre-validation services by completing the appropriate templates:
For more information, please review FAQ Ep57.
Questions? Contact hit@health.ny.gov for program clarifications and details.
How can WIC help your Clientele?
The mission of the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) is to safeguard the health of low-income women, infants, and children up to five years of age who are at nutrition risk. WIC provides participants access to nutritious foods to supplement diets, access information on healthy eating, and referrals to healthcare as well as non-healthcare services.
Applicants are determined adjunctively eligible for WIC if they receive SNAP, Medicaid, or Temporary Assistance for Needy Families (TANF). Alternatively, participants may be eligible based on their income. Participants must have a household income at or below 185% of the federal poverty line. Some working families may qualify: in 2016 a family of four with a household income of $44,955 could be eligible to receive WIC (http://www.health.ny.gov/prevention/nutrition/wic/income_guidelines.htm). An initial health assessment is conducted and then counseling is tailored to the specific health condition of each participant. This can be either dietary or medically-based.
WIC offers benefits such as breastfeeding support with 300 breastfeeding peer counselors on staff in New York State, nutrition education from registered dietitians and referrals to other healthcare services. Each food package provided to a participant is based on the Institute of Medicine recommendations and helps to achieve healthy body weights for infants and their mothers. WIC benefits save participants an average of $65 per month. By 2019, electronic benefits (eWIC) will be implemented which will replace paper checks and give more flexibility for shopping.
WIC not only benefits families, it also helps the community and helps to reduce healthcare costs. WIC has been shown to improve critical health outcomes through nutrition and breastfeeding counseling, access to fresh fruits and vegetables and referrals to healthcare and other critical social support. Prenatal WIC participation is associated with lower infant mortality rates. Wic moms have longer pregnancies resulting in fewer premature births. Since 2009 in New York State, obesity rates dropped 6% among one-year-olds and 3% in two-to-four-year-olds enrolled in WIC (1). Every WIC dollar spent on pregnant women saves $1.92-$4.21 in Medicaid costs for newborns and their mothers during the first 60 days of an infant's life (2).
Questions? Call the Growing up Healthy Hotline at 1-800-522-5006 or e-mail NYSWIC@health.ny.gov.
- 1Jackson, M., 2015. Early childhood WIC participation, cognitive development and academic achievement. Social Science and Medicine. 126, 145-153.
- 2United States Department of Agriculture (USDA) report: The savings in Medicaid costs for newborns and their mothers from prenatal participation in the Wic program. Addendum October 1991 - USDA is currently working on research to update the health care cost savings.
New York Medicaid Management Information System (NYMMIS) Continuous Improvements to Training
To better serve the provider community, enhancements are currently being made to all NYMMIS learning content and educational materials. As a result, all provider trainings currently scheduled are being postponed. Once the content and material changes are completed, a revised training schedule will be posted and further information will be provided in a future Medicaid Update. For additional information about NYMMIS visit the interim web site at www.interimNYMMIS.com.
Incontinence Supply Management Program: September 1, 2016
Social Services Law Section § 365-a (2)(g)(v) authorizes the Commissioner of Health to implement an Incontinence Supply Management Program. This initiative seeks to improve the quality of incontinence products provided to all Medicaid enrollees by establishing minimum quality standards for adult and youth size diapers and reduce costs for incontinence products while maintaining the existing provider (Durable Medical Equipment and Pharmacy) network. Incontinence supplies are covered by Medicaid for individuals aged 3 years and older and include medically necessary disposable and reusable diapers, liners and underpads.
Beginning September 1, 2016, all adult and youth sized diapers dispensed to all Medicaid enrollees must meet the following minimum product specifications established by the Department:
- No plastic (non-breathable) backed products;
- Rewet rate of <2.0 g;
- Rate of Acquisition (ROA) of <60 seconds;
- Retention capacity of >250 g;
- Presence of breathable zones with a minimum value of >100 cubic feet per minute (cfm)
- Presence of a closure system which allows for multiple fastening and unfastening occurrences.
These minimum standards apply to all adult and youth sized products dispensed to Medicaid enrollees residing in community settings by fee-for-service, managed care and managed long term care Medicaid providers.
To achieve this goal, the Department has awarded a preferred vendor supply contract to Twin Med, LLC for incontinence products to be purchased by Medicaid enrolled providers. Medicaid providers who purchase incontinence supplies from Twin Med will receive competitive pricing as defined by the contract and a defined formulary that meets the new minimum product specifications. Additional information will be provided in the coming months on formulary and pricing.
Providers will continue to be able to purchase incontinence products from alternative suppliers as long as the products provided meet the established minimum quality standards. Medicaid enrolled providers are not required to verify incontinence product quality standards if the products are purchased from Twin Med, LLC. Medicaid enrolled providers who choose to purchase incontinence supplies from an alternative supplier, however, are responsible for ensuring that any product dispensed meets the minimum product specifications established by the Department. The Department will incorporate review of incontinence product minimum quality specifications into routine pre-payment and post-payment reviews and other routine audit processes. The Department will also investigate any complaints received from Medicaid enrollees or other parties concerning Medicaid enrolled providers dispensing incontinence products which do not meet the established minimum product specifications.
Questions? Please visit the preferred distributor's New York website at www.twinmedny.com or contact the Bureau of Medical Review at 1-800-342-3005 or by email at OHIPMEDPA@health.ny.gov.
New Law in Relation to the Consumer Directed Personal Assistance Program (CDPAP) - Clarification
This article clarifies the policy set forth in the March 2016 Medicaid Update article titled, "New Law in Relation to the Consumer Directed Personal Assistance Program (CDPAP)." The March 2016 Medicaid Update article notified providers of changes that modified who can work as a CDPAP personal assistant for an eligible participant.
The purpose of the new law (Chapter 511 of the Laws of 2016) was to permit parents of adult children (age 21 or older) to be hired and work as their adult children's CDPAP personal assistants. The law was intended solely to expand the pool of who can be a CDPAP aide to include parents of adult children. It was not intended to narrow the pool of who can be a CDPAP aide.
When family members are hired as the personal assistant under the CDPAP, ask the following questions:
- Are the consumer and the personal assistant related?
- If yes, do they live in the same residence? If the answer is yes, be advised that the provision of CDPAP could still be allowed.
The parent of a child younger than 21 cannot be that child's aide nor can the spouse of a consumer be that consumer's personal assistant. A consumer's designated representative, regardless of the consumer's age, cannot be that consumer's personal assistant.
Living in the same home with the consumer does not disqualify a family member from being selected and hired as a personal assistant. Whether the consumer is self-directing or not is irrelevant. Provisions for the CDPAP have always allowed otherwise eligible family members to provide care while living in the same residence with the consumer. This remains unchanged in light of the April 1, 2016 change to the law.
Questions? Please contact the Division of Long Term Care at (518) 474–5888 .
Patient Centered Medical Home Statewide Incentive Payment Program - Reminder
Plan Types Eligible for Incentive Payments
The New York Medicaid Statewide Patient Centered Medical Home (PCMH) Incentive Payment Program provides financial incentives for practices that become recognized as a PCMH by the National Committee of Quality Assurance (NCQA) based on the standard year and level of achievement. Only specific public insurance products are included in the Statewide PCMH Incentive Payment Program; incentives are only given to practices for Medicaid enrollees who do not have Medicare or other third party coverage. The table below summarizes which public and Child Health Plus (CHP) product types are included in this program.
| Product Type | Eligibility for PCMH Incentive |
|---|---|
| Medicaid Managed Care (MMC) | Eligible |
| Medicaid Fee For Service (FFS) | Eligible |
| Child Health Plus (CHP) | Eligible |
| HIV Special Needs Plans (HIV SNPs) | Eligible |
| Health and Recovery Plans (HARPs) | Eligible |
| Essential Plans (EPs) | Ineligible |
| Qualified Health Plans (QHPS) | Ineligible |
| Managed Long Term Care (MLTC) | Ineligible |
| Program of All-Inclusive Care for the Elderly (PACE) | Ineligible |
For additional information on incentive amounts, see page 8 of the November 2015 issue of the Medicaid Update, titled "Patient Centered Medical Home Statewide Incentive Payments."
Questions/Information:
- For more information on how to achieve NCQA PCMH recognition, providers may contact NCQA at (888) 275–7585 or visit the NCQA website at www.ncqa.org
- MMC PCMH questions may be directed to the Division of Health Plan Contracting and Oversight at 518-474-5050, or the eMedNY call center at (800) 343–9000 .
- Medicaid FFS questions may be directed to: pcmh@health.ny.gov.
- For more information on claim eligibility please contact eMedNY at (800) 343–9000.
Guidance for New York State Medicaid Enrolled Laboratories Performing Testing for BRCA Mutations
The purpose of this communication is to call attention to changes in the New York State Medicaid Laboratory Procedure Code manual surrounding genetic testing for BRCA mutations. Laboratory manuals can be found at the following link:
https://www.emedny.org/ProviderManuals/Laboratory/index.aspx
BRCA - Testing for mutations in the BRCA1 and BRCA2 genes of individuals at high risk for hereditary breast and ovarian cancer may be billed using one of the following codes: 81162, 81211, 81212, 81214, 81215, 81216, or 81217 if the patient meets New York State Medicaid criteria. Please view the current guidelines, which were published in the October 2015 Medicaid Update, at the following link:
http://www.health.ny.gov/health_care/medicaid/program/update/2015/2015-10.htm
BRCA1 and BRCA2 mutation testing in conjunction with BRCA Large Rearrangement Test (BART) must be billed using CPT code 81162 effective 4/01/2016.
BART tests for large rearrangement mutations in BRCA genes. If a Medicaid enrollee previously had testing for BRCA1 and BRCA2 genes (CPT code 81211) with negative test results, and Bart testing was not performed, the enrollee may have BART only testing (represented by CPT code 81213). The addition of BART testing must be considered medically necessary.
For a Medicaid enrollee where BRCA1 and BRCA2 testing is being ordered for the first time, BART is performed as a reflex test if the BRCA1 and BRCA2 test results are negative. When performing both tests, CPT Code 81162 must be billed.
Questions regarding Medicaid fee-for-service policy may be directed to the Division of Program Development and Management at(518) 473–2160 . Questions regarding Medicaid Managed Care reimbursement and/or documentation requirements should be directed to the enrollee's Medicaid Managed Care plan.
New York State Medicaid Managed Care Pharmacy Benefit Information Website Update
Please note the new Uniform Resource Locator (URL) to the New York State Medicaid Managed Care Pharmacy Benefit Information Website: http://mmcdruginformation.nysdoh.suny.edu/. Users accessing the site with the old URL will be redirected.
The New York State Department of Health in partnership with the State University of New York at Stony Brook continue to add new features to the New York State Medicaid Managed Care Pharmacy Benefit Information Website. The most recent update, in May 2016, includes a MMC News banner that highlights the latest additions and features to the website, a contact us tab as well as the addition of Quantity Limits to specific drug files.
The contact us tab provides contact information for the Medicaid Helpline, helpful links to the Medicaid program as well as an email contact feature. This feature allows users to email NYSDOH feedback, comments or suggestions about the website.

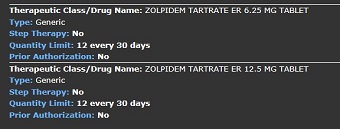
Health plans may limit quantity and dosage frequency consistent with how the Federal Drug Administration (FDA) has approved the use of the drug. Some quantity limits will be listed in the Quantity Limit field on the individual drug look up or in the covered drug drop down box under the drug look-up lists. In some instances, for chronic medications, the quantity limit field will say “none”. In this case the quantity limit will be consistent with the FDA daily dosing recommendation and plan monthly limits.
Individual Drug Look-up:
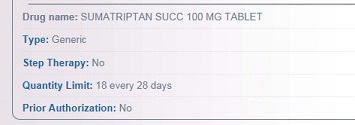
Drug Look-up List, click on "c":
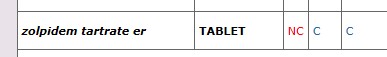
Results
The Medicaid Managed Care Pharmacy Benefit Information website is available at: http://mmcdruginformation.nysdoh.suny.edu/. Note this is a new URL. Users accessing the site with the old URL will be redirected.
In addition you can link to the website from the following pages:
New York State Department of Health Medicaid Managed care Page:
http://www.health.ny.gov/health_care/managed_care/
Click on Medicaid Managed Care Pharmacy Benefit Information Center
The eMedNY home page under "Featured Links" at:
https://www.emedny.org/index.aspx
Click on New York State Medicaid Managed Care Pharmacy Benefit Information Center
Redesigning New York's Medicaid Program Page under supplemental information on specific Mrt proposals:
http://www.health.ny.gov/health_care/medicaid/redesign/
Click on MRT 11 & MRT 15, Pharmacy Related Proposals and then click on Managed Care Plan Pharmacy Benefit Manager and Formulary Information
A Message from the New York City Health Department to Community Pharmacists in New York City
As a community pharmacist, you are among the most accessible and trusted healthcare professionals in your community. We at the New York City Department of Health and Mental Hygiene (NYC DOHMH) recognize that you serve a critical role not only by providing medications but also by providing important health information to residents of your communities on a daily basis.
NYC DOHMH is very interested in working with community pharmacists to support them in preparing for and recovering from disasters and other emergencies. However, we currently have no mechanism to communicate effectively with the more than 2000 independent community pharmacies in New York City.
To address this gap, NYC DOHMH created the Public Health Emergency Response Network Pharmacy Program (PHERN PP), a simple application that allows New York City pharmacies to quickly and easily "register" and provide pharmacy contact and other service information. This information will assist us in engaging in a productive, reciprocal exchange of information that is essential for effective emergency preparedness and response, and will enable us to better support you and your important work ensuring the health and well-being of all New Yorkers.
The registration process is simple and should only take 5 minutes. Go to http://on.nyc.gov/phern and scroll down to select the PHERN PP. Questions about the program can be directed to Eric Medina, PHERN PP Coordinator, at PHERNPP@health.nyc.gov. Thank you for supporting this important emergency preparedness initiative in New York City!
OPRA Prescription Requirements for Unlicensed Residents, Interns and Foreign Physicians in Training
As of March 25, 2011, Federal Rules and Regulations required that all ordering and referring physicians or other professionals providing services to Medicaid enrollees be enrolled as participating providers. In December 2013, New York State Medicaid issued a Special Edition of the Medicaid Update to provide enrollment requirements and guidance for all Ordering, Prescribing, Referring, and Attending (OPRA) servicing/billing providers. The purpose of this article is to provide a reminder regarding OPRA prescription requirements for unlicensed residents, interns and foreign physicians in training only.
- New York State Medicaid recognizes prescriptions written by providers legally authorized to prescribe per New York Education Law, Article 131, Section 6526, and 10 NYCRR 80.75(e). This includes unlicensed residents, interns and foreign physicians in training programs, under the supervision of a New York State Medicaid enrolled physician.
- In accordance with New York Education Law, Medicaid does NOT require the name and signature of the supervising physician to be included on the prescription. However, in order to enable billing by the dispensing pharmacy, prescriptions written by unlicensed residents must include the National Provider Identifier (NPI) of the supervising/attending physician who is enrolled in Medicaid (see option 2 below regarding billing requirements).
- New York State Medicaid only enrolls licensed providers. As a result, unlicensed residents, interns or foreign physicians in training programs are not eligible for enrollment as Medicaid providers.
- Effective January 2014, New York State fee-for-service Medicaid implemented claims editing that enforced the OPRA requirement for healthcare professionals, practice managers, facility administrators, and servicing/billing providers. Therefore, pharmacy claims for services ordered by unlicensed residents, interns and foreign physicians in training programs reject when initially submitted for payment. The following two (2) options continue to be available to pharmacies, to enable payment for unlicensed residents, interns and foreign physicians in training only:
- Resubmit the claim, using the NPI of the enrolled Medicaid provider (the intern or resident's supervising physician).
- In the event the NPI number of the supervising physician cannot be obtained or the pharmacy's billing system is limited to submitting only one prescriber NPI number, then use the urgent/emergency override option (outlined below).
Directions for Urgent/Emergency Override:
If you have a prescription written by an unlicensed resident, intern or foreign physician in a training program you will receive a reject code of "56" via NCPDP transaction stating the provider has a non-matched Prescriber Id listed in NCPDP field number 511-FB.
In the case of claims for items prescribed by unlicensed residents, interns or foreign physicians in training programs, pharmacies are allowed to provide the medication and receive reimbursement by resubmitting the claim using the following emergency override procedure:
- In the Reason for Service Code Field (439-E4) also known as the Drug Utilization Conflict Field – enter "PN" (Prescriber Consultation)
- In the Result of Service Code Field (441-E6) – enter one of the following applicable values (1A, 1B, 1C, 1D, 1E, 1F, 1G, 1H, 1J, 1K, 2A, 2B, 3A, 3B, 3C, 3D, 3E, 3F, 3G, 3H, 3J, 3K, 3M, 3N, or 4A)
- In the Submission Clarification Code Field (420-DK) also known as the Drug Prescription Override Field – enter "02" (Other Override)
Please note that the above override should not be used for a licensed prescriber who has not yet enrolled in Medicaid. In the event of a prescription being sent by a non-enrolled licensed prescriber, the prescriber should be encouraged to enroll in the Medicaid Program. Information regarding how to enroll can be found at: https://www.emedny.org/info/ProviderEnrollment/index.aspx
Contact the eMedNY Call Center at(800) 343–9000 for questions regarding this billing requirement.
The Medicaid Update is a monthly publication of the New York State Department of Health.
Andrew M. Cuomo
Governor
State of New York
Howard A. Zucker, M.D., J.D.
Commissioner
New York State Department of Health
Jason A. Helgerson
Medicaid Director
Office of Health Insurance Programs
Provider Directory
Office of the Medicaid Inspector General:
For suspected fraud complaints/allegations, call 1-877-87FRAUD, (877) 873-7283, or visit www.omig.ny.gov.
Provider Manuals/Companion Guides, Enrollment Information/Forms/Training Schedules:
Please visit the eMedNY website at: www.emedny.org.
Providers wishing to listen to the current week's check/EFT amounts:
Please call (866) 307-5549 (available Thursday PM for one week for the current week's amount).
Do you have questions about billing and performing MEVS transactions?
Please call the eMedNY Call Center at (800) 343-9000.
Provider Training:
To sign up for a provider seminar in your area, please enroll online at: http://www.emedny.org/training/index.aspx. For individual training requests,call (800) 343-9000.
Enrollee Eligibility:
Call the Touchtone Telephone Verification System at (800) 997-1111.
Medicaid Prescriber Education Program:
For current information on best practices in pharmacotherapy, please visit the following websites: http://www.health.ny.gov/health_care/medicaid/program/prescriber_education/presc-educationprog and
http://nypep.nysdoh.suny.edu/home
Need to change your address? Does your enrollment file need to be updated because you have experienced a change in ownership? Do you want to enroll another NPI? Did you receive a letter advising you to revalidate your enrollment?
Visit http://www.emedny.org/info/ProviderEnrollment/index.aspx and choose the link appropriate for you (e.g., physician, nursing home, dental group, etc.).
Medicaid Electronic Health Record Incentive Program questions?
Contact the New York Medicaid EHR Call Center at (877) 646-5410 for assistance.
Comments and Suggestions Regarding This Publication?
Please contact the editor, Amy Siegfried, at medicaidupdate@health.ny.gov